
Insights+: EMA Marketing Authorization of New Drugs in March 2023
Shots:
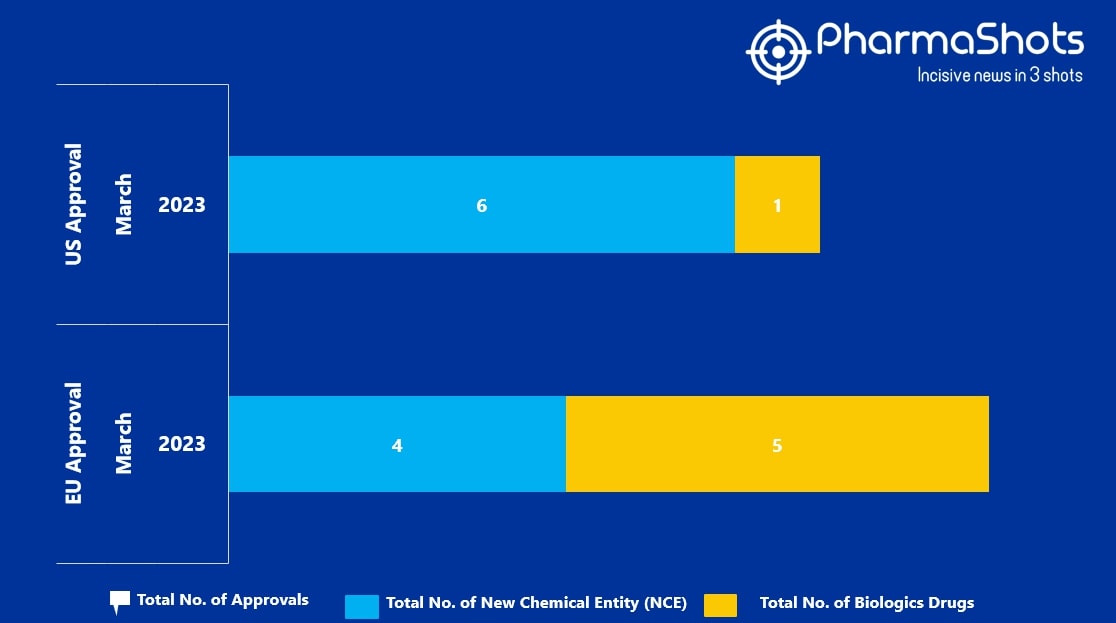
- The EMA approved 4 New Chemical Entity (NCE) and 5 Biologic Drugs in March 2023, leading to treatments for patients and advances in the healthcare industry
- In March 2023, the major highlights drugs were Reblozyl’s Approval for anemia in adult patients with non-transfusion-dependent beta thalassemia, Pombiliti for late-onset pompe disease
- PharmaShots has compiled a list of a total of 9 new drugs approved by the EMA in March 2023
1. Orion’s Darolutamide Receives EC’s Marketing Authorisation for the Treatment of Prostate Cancer
Nubeqa
Active ingredient: darolutamide Approved: March 01, 2023
Company: Orion Disease: Prostate Cancer
- The EC has granted marketing authorization for darolutamide + ADT in combination with docetaxel to treat patients with mHSPC
- The approval was based on the P-III trial (ARASENS) evaluating darolutamide (600mg, BID) + ADT and docetaxel vs ADT + docetaxel in a ratio (1:1) in 1306 patients with mHSPC. Darolutamide is developed jointly by Orion and Bayer
- The results showed a 32.5% reduction in risk of death with consistent benefits across clinically relevant 2EPs, the overall incidence of TEAEs was similar b/w treatment arms. Darolutamide was approved under the brand name “Nubeqa” in 80+ countries globally incl. the US, EU, Japan & China for nmCRPC & was also approved for mHSPC in a no. of markets incl. the US & Japan
Reblozyl
Active ingredient: luspatercept Approved: March 06, 2023
Company: BMS Disease: Non-Transfusion-Dependent Beta Thalassemia
- Reblozyl has received full marketing authorization from the EC to treat adult patients with anemia associated with non-transfusion-dependent beta-thalassemia. Reblozyl is being developed & commercialized through a collaboration with Merck
- The approval was based on the P-II study (BEYOND) evaluating Reblozyl vs PBO in a ratio (2:1) in 145 adults. The results showed 77.1% vs 0% of patients achieved the study’s 1EPs, ≥1.0 g/dL mean Hb increase from baseline
- In the 2EPs i.e., 49.0% vs 0% achieved a mean Hb increase of ≥1.5 g/dL over baseline from 37-48 wk. in the absence of transfusions, 89.6% vs 67.3% remained transfusion-free @1-24wk., improvements in patient-reported QoL outcomes were observed to correlate with Hb increases, serious AEs (11.5%)
Dupixent
Active ingredient: dupilumab Approved: March 21, 2023
Company: Regeneron Disease: Atopic Dermatitis
- The EC has approved Dupixent for sev. AD in children aged 6mos. to 5yrs. The approval was based on the P-III trial evaluating Dupixent (200/300mg, q4w) + low-potency TCS vs TCS alone in 162 children aged 6mos. to 5yrs.
- The results showed that 46% vs 7% of patients achieved ≥75% improvement in overall disease severity, 14% vs 2% achieved clear or almost clear skin & 55% vs 10% reduction in overall disease severity from baseline & 42% vs 1% reduction in itch from baseline
- Improvement in sleep quality, skin pain, and health-related QoL were seen in an overall and sev. populations while long-term efficacy data showed the clinical benefit at 16wks. sustained through 52wks. The safety results were consistent with the known safety profile of Dupixent
Hansizhuang
Active ingredient: serplulimab Approved: March 23, 2023
Company: Henlius Disease: Extensive-Stage Small Cell Lung Cancer
- The EMA has validated the application for Hansizhuang (serplulimab) in combination with CT (carboplatin and etoposide) for adults with ES-SCLC
- The application was based on the results from the P-III study (ASTRUM-005) evaluating the efficacy and adverse event profile of serplulimab + CT vs PBO + CT in 585 patients at 128 sites in multiple countries incl. China, Poland, Turkey, and Georgia. The results were presented at ASCO 2022 & were then published in the JAMA
- Hansizhuang, an anti-PD-1 mAb received ODD from the US FDA & EC for the treatment of SCLC. The therapy was also approved in China for the treatment of MSI-H solid tumors, sq. NSCLC and ES-SCLC
Pombiliti
Active ingredient: cipaglucosidase alfa Approved: March 27, 2023
Company: Amicus Therapeutics Disease: Late-Onset Pompe Disease
- The EC has approved Pombiliti (cipaglucosidase alfa) for the treatment of adults with late-onset Pompe disease (LOPD) used in combination with miglustat. The EMA’s CHMP opinion for miglustat is expected in Q2’23
- The approval was based on the results from the P-III (PROPEL) evaluating the efficacy, safety, and tolerability of Pombiliti
- Additionally, AT-GAA showed improvements in both musculoskeletal and respiratory measures in clinical studies. AT-GAA is a two-component investigational therapy that combines cipaglucosidase alfa to enable high-affinity uptake through the M6P receptor
Libtayo
Active ingredient: cemiplimab Approved: March 29, 2023
Company: Regeneron Disease: Non-Small Cell Lung Cancer
- The EC has approved Libtayo in combination with Pt-based CT for adult patients with advanced NSCLC with ≥1% PD-L1 expression & with no EGFR, ALK, or ROS1 aberrations
- The approval was based on the P-III trial (EMPOWER-Lung 3) evaluating Libtayo (350mg) + Pt-doublet CT vs Pt doublet CT alone in a ratio (2:1) in 466 patients which showed improvements across 1EPs & 2EPs incl. OS, 70% expressing PD-L1 ≥1%, m-OS (22 vs 13mos.) representing a 45% relative reduction in risk of death, m-PFS (9 vs 6mos.), ORR (48% vs 23%), m-DoR (16 vs 5mos.)
- Permanent treatment discontinuation due to AEs (5%) & the trial's primary analysis results were published in Nature Medicine. Libtayo was approved in the EU & other countries for advanced BCC, CSCC, NSCLC & cervical cancer
Entresto
Active ingredient: sacubitril and valsartan Approved: March 31, 2023
Company: Novartis Disease: Heart Failure
- The EMA’s CHMP has adopted a positive opinion recommending approval of Entresto to treat symptomatic chronic HF with LV systolic dysfunction in pediatric patients aged 1-<18yrs.
- The opinion was based on the 52wk. results from the P-III trials (PANORAMA-HF) & (PARADIGM-HF) showed similar reductions from baseline in the cardiac biomarker NT-proBNP in adult & pediatric patients, numerically better improvements from baseline on a no. of clinically relevant EPs over enalapril
- The safety & tolerability were consistent with that observed in adult patients. If Entresto is approved, it will become available to children with a new age-appropriate formulation to enable accurate & convenient administration
Pedmarqsi
Active ingredient: sodium thiosulfate Approved: March 31, 2023
Company: Fennec Disease: Ototoxicity
- The EMA’s CHMP has issued a positive opinion recommending the marketing authorization of Pedmarqsi (sodium thiosulfate) in the US for the prevention of ototoxicity (hearing loss) induced by cisplatin CT in patients aged 1mos. to <18yrs. with localized, non-metastatic, solid tumors
- The opinion was based on the 2 P-III trials (SIOPEL 6) and (COG Protocol ACCL0431) evaluating Pedmarqsi + cisplatin-based regimen vs cisplatin-based regimens alone. The results from both studies showed that the hearing loss incidence rate was lower in the Pedmarqsi + cisplatin arm vs cisplatin alone (28.6% vs 56.4%) & (35.1% vs 67.3%)
- The EC will review the CHMP's recommendation & issue a decision on Pedmarqsi’s approval by early June 2023. Pedmarqsi is currently marketed as Pedmark in the US
Briumvi
Active ingredient: ublituximab Approved: March 31, 2023
Company: TG Therapeutics Disease: Multiple Sclerosis
- The EMA’s CHMP has issued a positive opinion recommending the approval of Briumvi for adult patients with RMS with active disease defined by clinical or imaging features. The EC’s decision is expected in ~2mos.
- The opinion was based on the P-III trials (ULTIMATE I & II) evaluating Briumvi vs teriflunomide in 1094 patients across 10 countries. The trials were led by Lawrence Steinman, MD, Zimmermann Professor of Neurology & Neurological Sciences, and Pediatrics at Stanford University
- The results showed superiority over teriflunomide in reducing the ARR, the no. of T1 Gd-enhancing lesions & new or enlarging T2 lesions. The results were published in the NEJM. The centralized marketing authorization was valid in all EU MEMBER States, Iceland, Norway & Liechtenstein
Note: Entresto, Pedmarqsi & Briumvi received EMA’s CHMP Positive Opinion & EC’s Conditional Marketing Authorization for Darolutamide while EMA’s Validation of MAA for Hansizhuang (serplulimab)
Related Post: Insights+: EMA Marketing Authorization of New Drugs in February 2023
Tags

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.














